Which Statement Best Describes an Oxidation Reduction Reaction
In redox reactions a reduced half and an oxidized half occur together. Zn s Cu2 aq -- Zn2 aq Cu s.

Solved Which Statement Best Describes The Following Chegg Com
Which statement describes how electrons move if oxidation occurs on the left side of the cell and reduction occurs on the right side.

. Electrochemical Processes Cheat Sheet. Which step should be completed immediately after finding the oxidation states of atoms. Consider the following reaction.
The presence of which reactant is the best indicator of an oxidation-reduction reaction. This is the basis of redox reactions. Iron is being oxidized.
Which statement best describes the flow of electrons in a voltaic or galvanic cell. The chemical formula that shows the correct subscripts is D BeF₂. Which answer best describes what is happening in the following reaction.
A chemical reaction that involves oxygen. The two principal types are biological and natural. It permits the migration of ions In what kind of cell are the redox reactions made to occur by an externally applied electrical current.
The oxidation half-reaction occurs before the reduction half-reaction. This is a redox reaction in which octane C8H18 is oxidized. A Redox reaction oxidation-reduction reaction involves the exchangetransfer.
A chemical reaction in which there are fewer products than reactants. A chemical reaction that involves oxygen. Equations can be balanced by using the half-reaction method.
Which statement correctly describes a redox reaction. The correct statement that describes a Redox reaction is D. A chemical reaction in which there are fewer products than reactants.
Consider the half reaction below. C Iron transfers two electrons to copper. A chemical reaction in which electrons are released from the system.
But when an element is reduced it gains electrons. A chemical reaction in which electrons are released from the system. The type of reaction that is shown is.
Silver ion Ag is a stronger oxidizing agent than copper ion Cu2 and copper metal is a stronger reducing agent than silver. In todays world oxidation-reduction redox reactions play a significant role in determining the energy sources. Given that Cu 2HCI - Cu2 2CI- H2g has an overall reduction potential of -034 V what is a valid prediction about how this reaction works.
An oxidation-reduction redox reaction is a type of chemical reaction in which electrons are transferred between chemical species. C decomposition reaction. Which of the following is true about a redox reaction.
D Copper transfers two electrons to iron. This is a redox reaction in which octane C8H18 is oxidized. B Iron changes into copper.
In a redox reaction an electron is lost by the reducing agent. If there is formation of a precipitate the reaction is an oxidation-reduction reaction. E Two electrons are gained.
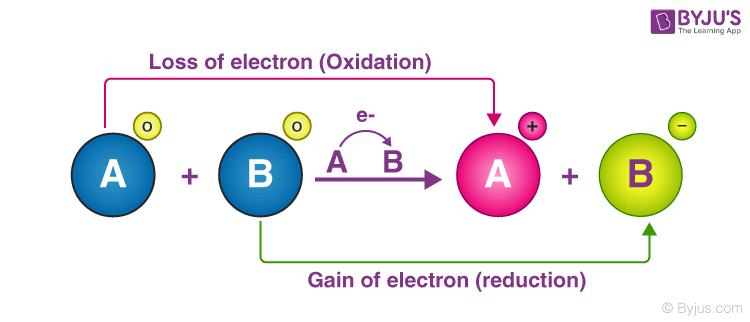
O O O If there is a change in the oxidation states of atorns from reactants to products the reaction is an oxidation- reduction reaction If there is formation of salt and water the reaction is an oxidation-reduction reaction. A chemical reaction in which electrons are transferred between reactants. The oxidation half-reaction and the reduction half-reaction occur simultaneously.
Fe Cu2 Fe2 Cu A Two electrons are lost. Which statement best describes what changes occur over the course of the following oxidation-reduction reaction. Chemistry 19092021 2310 NetherisIsTheQueen.
When an element is oxidized it loses electrons. 1Which statement best describes an oxidation-reduction reaction. The oxidation half-reaction occurs after the reduction half-reaction.
Which identifies an oxidation-reduction reaction. Electrons flow from the oxidation site to the reduction site through the wire. 1Which statement best describes an oxidation-reduction reaction.
Alternatively to get a lot of energy out of an oxidizing molecule hydrogen or oxygen will normally be removed. Third option is the correct one. Which answer best describes what is happening in the following reaction.
When an object is electroplated the occurrence of a redox reaction is nonspontaneous and it requires an electric current. Electrons move from left to right through M. Which statement best describes what is taking place in this half reaction.
Which of the following gets oxidized. However an oxidizing agent oxidizes something else and gets reduced therefore gaining electrons. Which statement best describes how a salt bridge maintains electrical neutrality in the half cells of an electrochemical cell.
Which statement best describes the oxidizing and reducing abilities of the reactants. Why Is The Redox Reaction Important In Photosynthesis.

Solved Which Statement Best Describes The Following Chegg Com
Is Cellular Respiration An Oxidation Or Reduction Reaction

1 Which Statement Best Describes An Oxidation Reduction Reaction 1 Point A A Chemical Reaction In Brainly Com
Comments
Post a Comment